SUMO is a small ubiquitin-like protein that is covalently attached to proteins. It is produced as a precursor protein, which is processed to its mature form by a SUMO-specific protease, Ulp1. SUMO is then activated by the formation of a thioester bond with Fub2, a component of the SUMO activating enzyme (E1). SUMO is then passed to a SUMO conjugating enzyme (E2). It can then be attached to target proteins. In some cases this requires the presence of a SUMO ligase (E3).
Sumoylation can affect protein localisation, enzymatic activity, protein-protein and protein-DNA interactions (Watts, 2013, Watts, 2007, Watts, 2006, Watts, 2004). It can also target proteins for ubiquitination and subsequent degradation for the proteasome. Through the analysis of mutants defective in sumoylation we have demonstrated that SUMO has multiple roles in maintaining genetic integrity. In addition, we have shown over the last few years, that several DNA replication, recombination and checkpoint proteins are SUMO modified e.g. Rad52 (Ho et al, 2001), Smc6 (Andrews et al, 2005), Rqh1, Mre11 (Watts 2008) and collaboratively, that Swi6, Taz1 and Tpz1 are also sumoylated (Shin et al., 2005, Spink et al, 2005, Garg et al, 2014). We have also analysed the role of sumoylation in meiosis in S. pombe (Spirek et al, 2010).
In the course of our work we also identified two S. pombe SUMO ligases, Nse2 and Pli1 (Andrews et al, 2005). Nse2 is a component of the Smc5/6 complex and is essential for viability. Pli1 is not essential for viability despite having dramatically reduced levels of sumoylation.
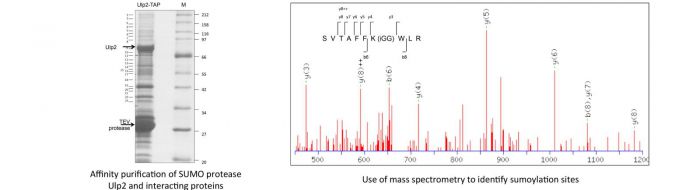
Many other proteins, in addition to those required for genetic integrity, are modified by SUMO or ubiquitin, included in these are translatin initiation factors, e.g. as reviewed in Watts et al 2014. Recently we have identified a number of translation initiation factors, such as eIF4G and eIF4A associated with the Ulp2 SUMO protease, some of which we have demonstrated to be sumoylated (Jongjitwimol et al., 2014).