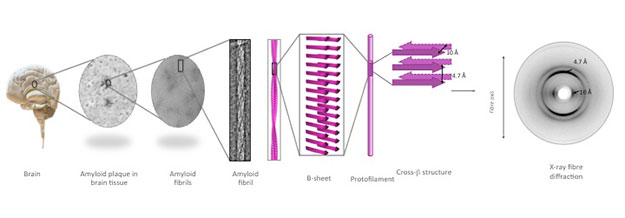
The Serpell Group work on the structure and function of amyloidogenic proteins using a range of biophysical and imaging techniques
Amyloid fibrils can be formed by a large number of proteins and peptides that share little sequence homology. Several diseases are associated with the deposition of amyloid fibrils in the tissues and each disease is associated with a different peptide or protein. Recently, it has become clear, that small intermediates of the fibril forming pathway can have a toxic effect on cells in vivo and are thought to play a central role in many of the amyloid diseases, particularly in Alzheimers disease. Amyloid fibrils have also been discovered fulfilling a functional role, lending their intrinsic strength and adhesive properties.
Our work centres around the formation of amyloid fibrils from disease-related and designed amyloidogenic peptides to explore the assembly mechanism and final structure of the amyloid fibril. We are exploring the effect of amyloidogenic oligomers and intermediates on neuronal cells and synthetic membranes aiming to better understand the process of neuronal toxicity associated with disease.