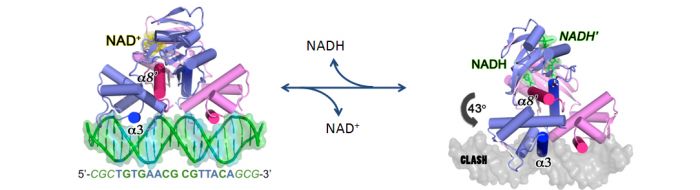
Sensing NADH/NAD+ redox state
Rex is a novel transcriptional repressor that is modulated by the cellular NADH/NAD+ ratio. Discovered by our lab in Streptomyces coelicolor, it is widespread in Gram-positive bacteria and is emerging as a key regulator of respiratory gene expression. Oxygen limitation causes an increase in cellular NADH/NAD+ ratio, which needs to be addressed to allow a continued source of NAD+ for further oxidation of food sources. Rex senses NADH, undergoes a major structural change, and disociates from its operator sites. This increases the expression of enzymes such as NADH dehydrogenase and cytochrome bd terminal oxidase, which restore redox balance.
Collaborator: Dr Clara Kielkopf, University of Rochester, USA.
RsrA – a redox sensing anti-sigma factor
The disulphide stress response sigma factor SigR is controlled by an anti-sigma factor RsrA, which binds to SigR and prevents it from interacting with RNA polymerase. A oxidative shift in the thiol-disulphide redox balance of the cell leads to RsrA oxidation by disulphide bond formation, which causes the dissociation of SigR, allowing the sigma factor to bind RNA polymerase. We are interested in the mechanism of disulphide-bond formation.
Collaborators: Prof Colin Kleanthous, University of York; Prof Mark Buttner, John Innes Centre.