Prior to joiing Sussex, I was interested in the use of hyperpolarisation and time-resolved NMR spectroscopy. Below is a brief overview of my work in these areas, with some key publications lists.
Sensitivity enhancement and hyperpolarisation
NMR is inherently insensitive. This is because the interaction of nuclear magnetic moments with an applied magnetic field is extremely small. The situation is dramatically worse for nuclei with low natural abundance of their magnetically-active isotopes, such as carbon-13 (1.1%), nitrogen-15 (0.37%) and silicon-29 (4.67%). I was interested in developing methods which can be used to improve the sensitivity of NMR spectroscopy, both as a tool for structure elucidation and to probe biologically interesting problems.
 Photo-CIDNP of bovine alpha-lactalbumin
Photo-CIDNP of bovine alpha-lactalbumin
Photo-Chemically Induced Dynamic Nuclear Polarisation is a technique whereby a spin-state selective chemical reaction is used to generate nuclear polarisation. In principle any reaction which proceeds via a radical pair intermediate can be used. Typically, we have employed the photochemical reaction between a flavin and the aromatic amino acid side chains of tyrosine, tryptophan and histidine. This nuclear polarisation has then been used to study protein folding and to probe the structure of partially folding or denatured proteins.
Dynamic Nuclear Polarisation is a technique nearly as old as NMR spectroscopy itself. It relies on the transfer of polarisation from an unpaired electron in a cryogenic solid to the nuclei of interest. This project was in collaboration with Oxford Instruments and Pfizer, Ltd. We have developed methods using this heteronuclear hyperpolarisation, such as the co-acquisition of carbon-13 and nitrogen-15 polarisation and the measurement of heteronuclear spin-lattice relaxation times, on a timescale only relying on the time required to generate the nuclear hyperpolarisation.
Key publications
- K. H. Mok, L. T. Kuhn, M. Goez, I. J. Day, J. C. Lin, N. H. Andersen, and P. J. Hore. A pre-exisiting hydrophobic collapse in the unfolded state of an ultrafast folding protein. Nature, 447(7140):106-109, 2007.
- K. H. Mok, T. Nagashima, I. J. Day, P. J. Hore, and C. M. Dobson. Multiple subsets of side-chain packing in partially folded states of α-lactalbumins. Proc. Natl. Acad. Sci. USA, 102(25):8899-8904, 2005.
- I. J. Day, J. C. Mitchell, M. J. Snowden, and A. L. Davis. Applications of DNP-NMR for the measurement of heteronuclear T1 relaxation times. J. Magn. Reson., 187(2):216-224, 2007.
- I. J. Day, J. C. Mitchell, M. J. Snowden, and A. L. Davis. Co-acquisition of hyperpolarised 13C and 15N NMR spectra. Magn. Reson. Chem., 45(12):1018-1021, 2007.
Time-resolved and stopped-flow NMR
NMR is one of the few analytical techniques which is capable of giving truly atomic resolution. I have been interested in developing NMR-based methods which enable kinetic information to be obtained, both for chemical and biochemical reactions.
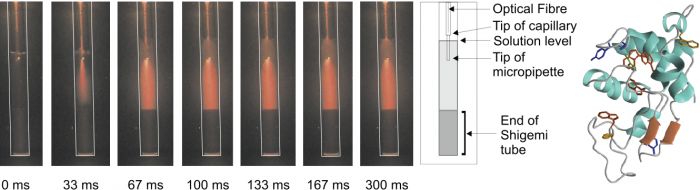
 Time-resolved imaging of a stopped-flow NMR injection
Time-resolved imaging of a stopped-flow NMR injection
An example of this has been the development of a stopped-flow mixing accessory for a standard NMR spectrometer. This device is capable of producing complete and homogeneous mixing within 50 ms following the injection event. This device has been used in a number of studies of protein folding. However, its utility is applicable to any binary mixing scheme.
I also have an interest in developing time-resolved NMR methods which provide access to kinetic parameters. This includes the modification of "traditional" NMR experiments to suit the systems under investigation.
Key publications
- K. H. Mok, L. T. Kuhn, M. Goez, I. J. Day, J. C. Lin, N. H. Andersen, and P. J. Hore. A pre-exisiting hydrophobic collapse in the unfolded state of an ultrafast folding protein. Nature, 447(7140):106-109, 2007.
- K. H. Mok, T. Nagashima, I. J. Day, J. A. Jones, C. J. V. Jones, C. M. Dobson, and P. J. Hore. Rapid sample-mixing technique for transient NMR and Photo-CIDNP spectroscopy: Applications to real-time protein folding. J. Am. Chem. Soc., 125(41):12484-12492, 2003.
- S. M. Harper, L. C. Neil, I. J. Day, P. J. Hore, and K. H. Gardner. Conformational changes in a photosensory LOV domain monitored by time-resolved NMR spectroscopy. J. Am. Chem. Soc., 126(11):3390-3391, 2004.