Research within the Crossley lab is directed toward the synthesis and study of reactive and electronically distinctive molecules from the interface of traditional transition metal and main group chemistry. Current work is focused in three general areas:
Conjugated Phosphacarbon Complexes
Low-coordinate phosphorus(III) compounds are somewhat unique in their ability to mirror many of the structural and reactivity features of classical carbon-based compounds, while also exhibiting an distinctly individual electronic and chemical nature. This is particularly apparent for phosphaalkenes (RP=CR'R'') and phosphalkynes (RC≡P), which serve as archetypes for the isolobal phosphorus-carbon analogy.
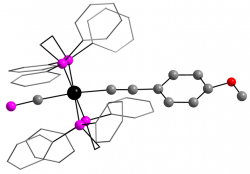
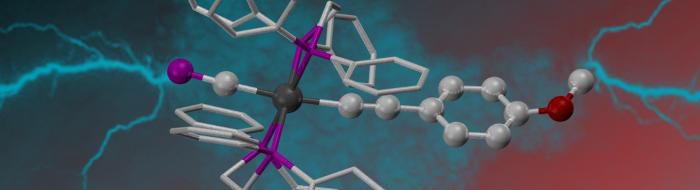
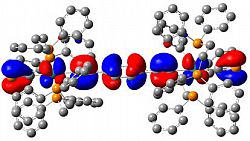 We are working to incorporate these fragments within organometallic systems that involve extended through-conjugation, thus ‘doping’ these carbon-rich ‘molecular wire’ models to modify their conductive responses. Our work toward this challenging goal has included isolation of the first complexes to feature the rare cyaphide (‘C≡P’) ligand as part of a metal-mediate through-conjugation with acetylenic chains, in both monometallic and bimetallic scaffolds.
We are working to incorporate these fragments within organometallic systems that involve extended through-conjugation, thus ‘doping’ these carbon-rich ‘molecular wire’ models to modify their conductive responses. Our work toward this challenging goal has included isolation of the first complexes to feature the rare cyaphide (‘C≡P’) ligand as part of a metal-mediate through-conjugation with acetylenic chains, in both monometallic and bimetallic scaffolds.
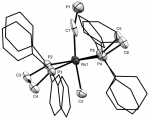
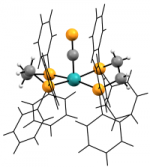 In seeking more facile routes to variation of the alkynyl fragment we expanded the range of cyaphide complexes to include trans-alkyl and halo systems, ultimately leading us to isolate the coordinately unsaturated complex cation [Ru(dppe)2(C≡P)]+. The first stably isolable complex featuring cyaphide within a flexible coordination sphere, this is a convenient starting point for our on-going investigations focussed on modifying the ancillary coordination sphere
In seeking more facile routes to variation of the alkynyl fragment we expanded the range of cyaphide complexes to include trans-alkyl and halo systems, ultimately leading us to isolate the coordinately unsaturated complex cation [Ru(dppe)2(C≡P)]+. The first stably isolable complex featuring cyaphide within a flexible coordination sphere, this is a convenient starting point for our on-going investigations focussed on modifying the ancillary coordination sphere
Phosphorus-containing Ligands
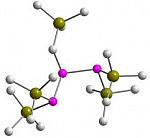 Phosphane ligands fulfil a diverse role in modern coordination and organometallic chemistry, whether in stabilising unusual coordination geometries, serving as labile ligands in pro-catalysts or affording asymmetric induction for catalytic processes.
Phosphane ligands fulfil a diverse role in modern coordination and organometallic chemistry, whether in stabilising unusual coordination geometries, serving as labile ligands in pro-catalysts or affording asymmetric induction for catalytic processes.
We are interested in developing novel and reactive ligand systems, particularly those that feature rare or unusual electronic functionalities. Our work in this area has included a focus on ligands incorporating phosphomide-type units, both in acyclic and, more recently, cyclic scaffolds. 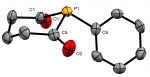 Our recently described phosphinane- and phosphepane-α-diones represent rare examples of saturated phosphacycles,
Our recently described phosphinane- and phosphepane-α-diones represent rare examples of saturated phosphacycles, 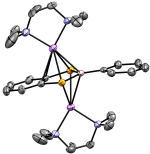 in which the flanking acyls effect appreciable stabilisation of the lone pair, affording weakly donating, sterically encumbering ligand sets, which we are investigating for catalytic applicability.
in which the flanking acyls effect appreciable stabilisation of the lone pair, affording weakly donating, sterically encumbering ligand sets, which we are investigating for catalytic applicability.
In other work we have we recently described the first diphosphaborolediide, which exhibits a hybridised phospholide/boroleide character and is an effective ligand for low-valent metal centres. We are currently exploring the chemistry of this novel ligand and its derivatives.
Phosphacyclophanes
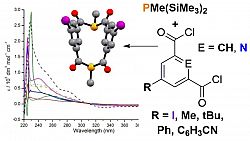 In an extension of our work with simple phosphomide-type ligands we have developed a family of diphosphametacyclophanes, which self-assemble from a simple condensation reaction between isophthaloyl chloride and RP(SiMe3)2. These macrocycles have been shown to act as bulky ligands, both through both monodentate coordination and bridging modes. More significantly, the presence of the diketophosphanyl moieties makes these molecules interesting from an opto-electronic standpoint. We have recently demonstrated the effect on both their electronic spectra and electrochemical responses of incorporating different substituents on the aromatic backbone and explored this computationally. The arene fragments were thus found to serve as insulators between the two diketophosphanuyl moieties; achieving control over this is a goal we are currently exploring
In an extension of our work with simple phosphomide-type ligands we have developed a family of diphosphametacyclophanes, which self-assemble from a simple condensation reaction between isophthaloyl chloride and RP(SiMe3)2. These macrocycles have been shown to act as bulky ligands, both through both monodentate coordination and bridging modes. More significantly, the presence of the diketophosphanyl moieties makes these molecules interesting from an opto-electronic standpoint. We have recently demonstrated the effect on both their electronic spectra and electrochemical responses of incorporating different substituents on the aromatic backbone and explored this computationally. The arene fragments were thus found to serve as insulators between the two diketophosphanuyl moieties; achieving control over this is a goal we are currently exploring