Mechanochemistry
In April 2019, IUPAC listed mechanochemistry as one of 10 chemistry technology innovations that will change the world. Inducing reactions through mechanical forces has the potential to avoid the use of solvents and facilitate processes that are hard to do by traditional methods. Traditional flasks and high temperature baths could be replaced with mixer mills (see Figure), planetary mills or solid extruders. However surprisingly few classic processes in heterocycle synthesis have been explored using mechanochemistry. We are investigating the use of this technology for the synthesis of pyridines and thiazoles.
Figure. The Retsch MM400 mixer mill uses stainless steel and zirconium oxide ball mill reactor vessels for mechanochemistry. We gratefully acknlowledge the European Regional Development Fund (ERDF) for funding LabFact under the lnterreg V Programme.


Chemical Intervention in Accelerated Ageing
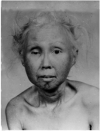
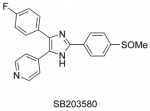
Werner’s Syndrome (WS) is a rare human genetic disease that shows accelerated ageing and an increased cancer incidence, and is studied for the insights that it might give into both processes. A key feature of WS is that cells from these individuals show a much reduced cellular lifespan in the laboratory as a consequence of replicative senescence. This project, which spans the chemistry-life sciences interface, seeks to investigate this phenomenon using different chemotypes prepared in our synthetic laboratories. This offers great potential for the development of clinical treatments for this disorder, in so doing understanding some of the fundamental mechanisms operating in telomere-independent cell senescence, cancer and human ageing and may lead to the first chemotherapeutic treatment for WS. Treatment of WS cells with p38 MAPK inhibitors such as SB203580 (see Figure 1) reverses the ageing phenotype and restores the replicative capacity (Pharmaceuticals 2010, 3, 1842).
Figure 1. Left-to-right: a WS patient at 15 years; 48 years; a p38 MAPK inhibitor; untreated WS cells; treated cells

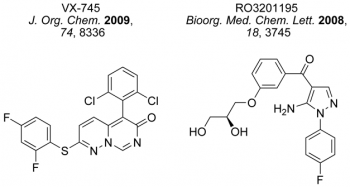
As part of a national New Dynamics of Ageing (ESRC) and BBSRC sponsored programmes, we have prepared a range of MAPK inhibitors (Figure 2) using microwave heating and have screened them in WS cells to understand the mechanisms that operate in cell ageing an stress-induced senescence. Further studies are underway to unravel the pathophysiology of this condition towards a clinical treatment.
Figure 2. Some inhibitor chemotypes prepared in our laboratories for study in WS cells
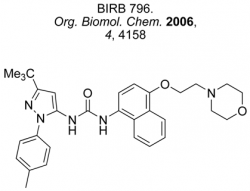
Synthesis of Thiopeptide Antibiotics
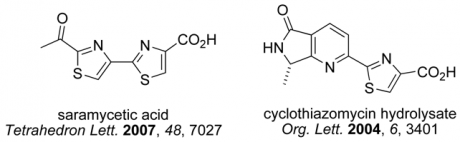
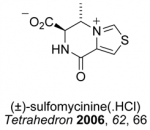
Infectious diseases have been estimated to kill almost 50,000 people every day and have emerged as the world’s leading cause of premature death. In this context, the search to discover, prepare and understand new antibiotics is an area of profound medical, social and political importance. The thiopeptide antibiotics represent promising candidates and leads for the development of new antibacterial agents effective against the so-called `superbugs’. As the search continues for new agents effective against resistant strains of bacteria, compounds that target the GTPase centre, such as the thiopeptide antibiotics, have been largely ignored by the pharmaceutical industry. We are developing new highly convergent synthetic approaches towards this family (see Figure 3) and related pyridine-containing natural products to verify structure, understand the mode of action and challenge our synthetic methods. Microwave-heating and multiple-component reactions have delivered a range of thiopeptide targets and their chemical derivatives in previous work within the group (Figure 4).
Figure 3. Pyridine-containing natural products such as the thiopeptide antibiotics
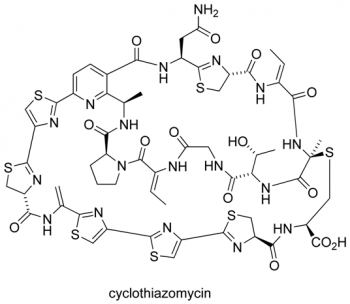
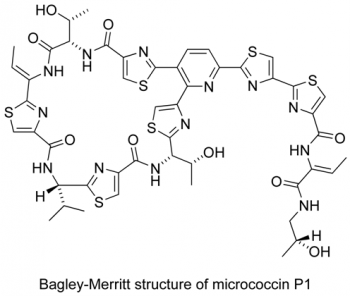
Figure 4. Some previous targets in the synthesis of thiopeptide antibiotics
New Methods and Technology for the Synthesis of Aromatic Heterocycles
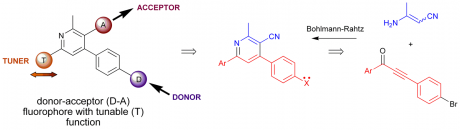
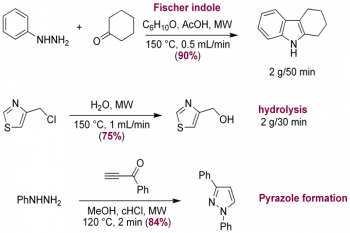
The last decade has seen renewed focus on the need to harness enabling to simplify processes in organic synthesis. Built upon recent advances in automation, principally driven by the needs of combinatorial chemistry, the realization that technological advances could open up new molecular space, streamline process efficiency and integrate synthetic operations with other necessary processes such as purification, analysis and biological evaluation has driven us to re-examine the age-old traditions of synthetic experimental design. Multicomponent reactions offer convenient procedures for the introduction of many points of structural diversity into heterocyclic compounds prepared in a straightforward manner in a single synthetic step. Combining these features with the extremely fast reaction kinetics of microwave-assisted organic synthesis provides new methods for the rapid and efficient synthesis of heterocyclic libraries suitable for biological evaluation and SAR studies. We are developing routes to pyridines, pyrimidines, thiazoles, indoles, pyrazoles and oxazoles using a combination of microwave irradiation, flow technology, multiple-component reactions and cascade processes (see Figure 5) for the rapid or continuous preparation of targets of interest, such as the multi-step Bohlmann-Rahtz pyridine synthesis, tandem oxidation-heteroannulation chemistries and transition metal mediated C-H functionalization reactions.
Figure 5. Left: a new continuous flow microwave reactor; right: heterocyclic chemistry in a microwave flow reactor

We are developing this technology for the synthesis of pyridines with interesting photophysical properties using the Bohlmann-Rahtz reaction (Figure 6; see Chem. Commun. 2009, 5165), preparing novel fluorophores and metal ion sensors (Dalton Trans. 2010, 39, 3163) with interesting solvatochromic behaviour based upon a cyanopyridine scaffold.
Figure 6. Solvatochromic behaviour (CHCl3 and DMSO) of donor-acceptor pyridines with photophysical tunability