Ultracold chemical reactions
Cold molecular ion species are of fundamental interest to chemists as well as physicists. The development of many different technologies such as ion trapping, the ability to decelerate molecular beams, and trapping neutral gases alongside ions has given rise to a vast new area of ultra-cold chemistry.
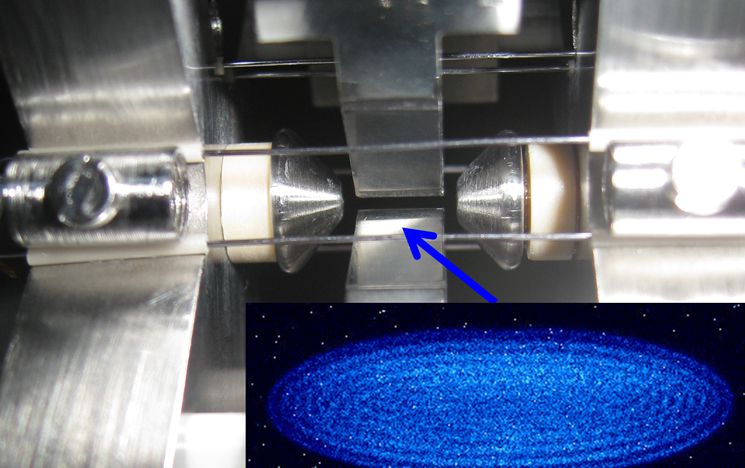
Trapping structure to confine target ions
While chemical reactions at temperatures well above absolute zero are governed only by entropy at very low temperatures, the dynamics is dominated by quantum mechanical effect such as quantum tunnelling.
In this ultra-cold regime, we now have unprecedented control over the experimental conditions: small, pure samples can be created under vacuum – almost completely sealed off from the environment. Reactions between just individual reactant molecules can even be achieved. Not only this, but we can also control the quantum states of the molecules: reaction rates can be tuned depending on the states of reactants.
In collaboration with the group of Prof. Softley (University of Oxford) we investigate reactions between Stark decelerated polar molecules and sympathetically cooled molecular ions embedded in a framework of laser cool calcium ions.
Using the crystal weighing technique, chemical reactions can be detected due to the associated change in the average mass of the ion crystal. Each reaction between a trapped ion in the crystal and a neutral species can be observed through a change in secular frequency. Due to the high precision of the method, the exact product of the reaction can be determined.