Research
We are interested in the identification and characterization of ncRNAs that are crucial regulators of cancer progression and to evaluate how this RNA class acts to mediate this role.
With the recent advances in next generation sequencing (NGS) technologies we now understand that the genome is not only transcribed to generate RNA molecules committed to produce proteins but also to create non-coding RNAs (ncRNAs) that have regulatory activities. This class of RNA can be further classified into long noncoding RNAs (lncRNAs) or microRNAs (miRNAs) depending on their length. Many ncRNAs are important regulators of genes that are crucial for cancer progression. However, the mechanism of action and role in cancer of thousands of ncRNAs still need to be characterised.
-
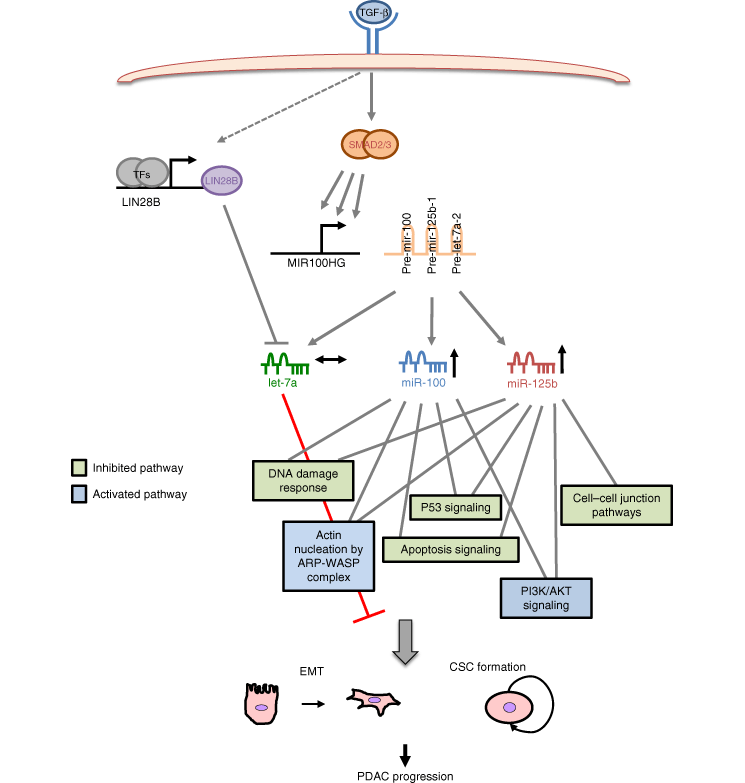
text description of the above diagram
The diagram shows that TGFb cytokine released from the tumour microenvironment interacts with its receptor on the surface of tumour cells to activate SMAD transcription factors. This signalling activates the transcription of MIR100HG which produces three precursor microRNAs: let-7a, miR-100 and miR-125b. However whereas let-7a maturation is impaired by LIN28B activity, the levels of miR-125b and miR-100 is induced to coordinate the TGFb response. This effect induces epithelial to mesenchymal transition (EMT) cancer stem cell (CSCs) formation which resutls in pancreatic adenocarcinoma (PDAC) progression. Pathways repressed by these miRNAs are particularly enriched by p53 signalling, apoptosis, cell-cell junctions and DNA responses. Whereas pathway activated include oncogenic Actin nucleation by ARP-WASP complex and PI3K/AKT signalling.
Usually, ncRNAs perform their action by binding to proteins, cis-regulatory genomic regions (enhancer and promoters) or other RNA molecules to modulate gene expression during cellular functions. Dysregulation of ncRNA expression can induce cancer progression. Therefore, it is crucial to characterise ncRNAs and establish their mechanism of action to be able to completely understand cancer and develop effective target-based therapy.
We principally focus our research on three different epithelial cancers: Pancreatic Cancer, Breast Cancer and Colon Cancer. These cancers are amongst the most common and are the ones that produce the highest level of mortality. In particular, despite intensive research, survival rate for pancreatic cancer has not changed for the last 50 years and still remains below 5%.
Currently we focus our research on the mechanism of TGF-beta-dependent induction of pancreatic cancer metastasis, through activation of oncogenic microRNAs, by performing NGS, bioinformatics and novel CRISPR-CAS9-based approaches in vitro and in vivo. The mechanism of TGF-beta-dependent induction of pancreatic cancer metastasis through regulation of novel nuclear long ncRNAs (lncRNAs) that act through modulation of enhancer promoter interactions.
The identification of chromatin associated lncRNAs involved in cancer stem cell formation. We particularly focus here on the identification of the gene transcriptionally regulated by both these lncRNAs and their interacting transcription factors, during cancer stem cell self-renewal, by performing CHART-seq and ChIP-seq approaches. Evaluation of the role of miRNAs and components of the miRNA biogenesis machinery in the cellular stress response and regulation of CAP-dependent and independent protein translation.
Identification of networks composed of protein coding genes and ncRNAs that specifically drive the different breast cancer subtypes (luminal A, luminal B, Basal, HER2-positive and normal-like) by applying genome editing screening approaches. Evaluation of the role of enhancer associated lncRNAs in cell proliferation of ER-positive breast cancers.
Publications

Please go to Dr. Leandro Castellano profile page for list of publications
Funding
Work in our laboratory is funded by grants from the Medical Research Council, Action Against Cancer, Biotechnology and Biological Sciences Research Council (BBSRC) and private companies.



