The aims of our current work are to investigate the nature and regulation of signalling pathways regulating mRNA utilisation in eukaryotic cells during proliferation and differentiation. In addition, we are developing tools and approaches to determine where in the cell specific protein synthesis is occuring. Although the regulation of protein synthesis is fundamental to cell growth and/or survival, relatively little is actually known about the role of phosphorylation of translation initiation factors in modulating this process.
Current research interests
Background
The initiation stage of translation is a major site of regulation of gene expression with strong analogies to the assembly of transcription complexes at promoter sites on DNA. In terms of regulatory significance, the second phase of initiation, the binding of mRNA to the ribosome, has the potential to modulate both the overall rate of protein synthesis and the selective recruitment of specific mRNAs for translation (Fig.1). This step can be modulated by both the phosphorylation of key initiation factors and by changes in the levels of these factors. The eIF4 initiation factors (eIF4E, eIF4A, eIF4B, eIF4G and the eIF4F complex) are responsible for recruiting mRNA to the ribosome. eIF4E interacts specifically with the mRNA cap structure and is essential for cap-dependent translation. eIF4A exhibits RNA-dependent ATPase and bidirectional RNA helicase activities,with the latter strongly enhanced by eIF4B. eIF4G, of which there are two forms (eIF4GI, eIF4GII), functions as an adapter protein in the assembly of the initiation complex, eIF4F. It binds, via a defined and conserved sequence, to the convex surface of eIF4E and has a centrally located domain which interacts with eIF3 and eIF4A, with consensus elements characteristic of RNA binding proteins; both mammalian eIF4G homologues possess an N-terminal binding site for poly(A) binding protein (PABP).
Work which has led up to the currect project
Of central importance to our understanding of how the translational machinery is activated in eukaryotic cells was my finding that, concomitant with increased rates of protein synthesis, there was an increase in the phosphorylation of eIF4E and its recovery into eIF4F complexes (Fig.2). The research carried out in my lab over the past 15 years, funded by a Senior Research Fellowship from The Wellcome Trust and research grants from the BBSRC, has been directed at the identification of the intracellular signalling mechanisms involved in the regulation of mRNA recruitment to the ribosome during different phases of the cell cycle. In particular, we have chosen to determine the role of individual initiation factors and their post-translational modification in the activation of protein synthesis following the induction of cell growth, and in the maintenance of translation rates during cell death and differentiation. My laboratory has made a wide range of important contributions to understanding the function of translation factors in mammalian cells. These relate to a number of different components of the translational machinery, including several initiation factors (eIF4E, eIF4G, PABP), a family of protein kinases with initiation factors as substrates (e.g. Mnk1), and the regulation of initiation factor localisation in cells under different growth conditions.
The research carried out in my lab over the past 17 years, funded by a Senior Research Fellowship from The Wellcome Trust and research grants from the BBSRC, has been directed at the understanding the importance of the intracellular signalling mechanisms involved in the regulation of mRNA recruitment to the ribosome during different phases of the cell cycle. In particular, we have chosen to determine the role of individual initiation factors and their post-translational modification in the activation of protein synthesis following the induction of cell growth, and in the maintenance of translation rates during cell death and differentiation. My laboratory has made a wide range of important contributions to understanding the function of translation factors in mammalian cells. These relate to a number of different components of the translational machinery, including several initiation factors (eIF4E, eIF4G, PABP), a family of protein kinases with initiation factors as substrates (e.g. Mnk1), and the regulation of initiation factor localisation in mammalian cells under different growth conditions.
In summary, our main achievements have been:
(a) eIF4E phosphorylation
In work funded by The Wellcome trust, we have made a number of important discoveries in this area:
(i) we demonstrated that the mTOR signalling pathway is not required for the acute phosphorylation of eIF4E in Xenopus oocytes [1] or primary mammalian cells [2].
(ii) we challenged the dogma that eIF4E was rate-limiting for translation [3].
(iii) we showed that the phosphorylation of eIF4E was increased in response to stress [4] and physiological activation of the T-cell receptor complex [5].
(iv) we demonstrated that eIF4E phosphorylation required indirect signalling through the ERK and/or p38 MAP kinase pathways [5-7].
(v) with Xenopus cells, we showed that, in contrast to mammalian cells, eIF4E phosphorylation occurred without any apparent change in the association of eIF4E with its inhibitory binding protein, 4E-BP1 [6,7].
(vi) we validated the use of the permeable inhibitor, SB203580, in the study of the phosphorylation of eIF4E in cultured cells using a line stably expressing a drug-resistant stress activated kinase [8].
(vii) we showed conclusively that the phosphorylation of eIF4E was not a pre-requisite for the activity of this protein in vitro or in vivo [9].
(viii) we determined that the phosphorylation of eIF4E was not required for de novo protein synthesis following recovery of cell from salt shock [10], but that this reflected a novel cross-talk between the MEK1/2 and mTOR signaling pathways in mammalian cells [11].
(b) eIF4F complex assembly and translational control
Using a combination of approaches to isolate eIF4E and its associated proteins, we conclusively showed that :
(i) serum stimulated the association of eIF4F and PABP in Xenopus kidney cells [6].
(ii) other proteins involved in regulating mRNA translation have a critical role in promoting the interaction of eIF4G with eIF3 [12].
(iii) using regenerating mouse liver, mTOR signaling in required for the assembly of eIF4F complex and cyclin D1 protein expression under physiological conditions [13].
(iv) the proteasome inhibitor, MG132, promoted a re-programming of translation and generation of a novel eIF4F complex containing hsp25 [14].
(v) the activation of Pak2, a kinase involved in the preventing cell growth and promoting cell death, results in the phosphorylation of eIF4G and decreased levels of eIF4F [15].
(vi) multiple forms of eIF4GI exists in human cells with different abilities to maintain protein synthesis rates [16,29].
(vii) isoforms of eIF4GI lacking individual domains are still capable of driving protein synthesis rates in vitro and in vivo [17,29].
(viii) Inhibition of mammalian target of rapamycin (mTOR) signalling in C2C12 myoblasts prevents myogenic differentiation without affecting the hyperphosphorylation of 4E-BP1 [30].
(c) Localisation of initiation factors in mammalian cells
In work funded by the BBRSC, we used novel antisera and Alexa-fluor coupled antibodies to conclusively demonstrate that:
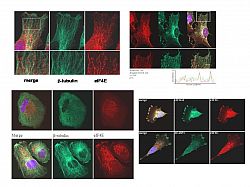
(i) a population of eIF4G actually resides in the nucleus where it is actively associated with the splicing machinery, providing a functional link between the nuclear and cytoplasmic cap-binding proteins [18].
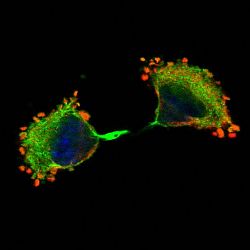
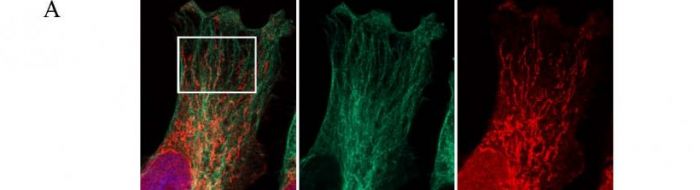
(ii) PABP co-localises with paxillin at the dense ER and the leading edges of migrating cells [19].
(iii) eIF4GI possesses a functional nuclear localization signal in its N-terminus responsible for the nuclear accumulation of an apoptotic fragment of eIF4G [20].
(iv) MG132 promotes the re-localisation of initiation factors in myoblasts in culture [21].
(v) Mnk1 actively shuttles into the nucleus where it might target a nuclear pool of eIF4E [22].
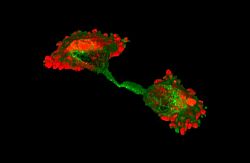
(vi) Ribosomes and translation initiation factors localise to talin/beta3-integrin-enriched adhesion complexes in spreading and migrating mammalian cells [31].
(d) Proteolytic cleavage of eIF4G during viral infection and cell death.
Over a number of years, our Wellcome Trust/BBSRC funded research has conclusively shown:
(i) translation of uncapped mRNA could be stimulated by the addition of purified, pre-cleaved eIF4G to the untreated reticulocyte lysate, with the C-terminus of eIF4G sufficient to support cap-independent translation [23].
(ii) binding of eIF4E to eIF4G altered eIF4G structure allowing cleavage by L protease [24].
(iii) eIF4G was cleaved during apoptosis [21] and we mapped these cleavage sites [25-27].
(iv) using caspase-deficient tumour cell lines, we showed the role for caspase-8, but not a requirement for p38MAPK signalling in eIF4G cleavage [28].
(v) caspases target the interaction between PABP and eIF4B to decrease protein synthesis during apoptosis [28].
[1]. Morley, S.J., and Pain, V.M. (1995). J. Cell Sci. 108, 1751-1760. [2]. Morley, S.J., Rau, M., Kay, J.E. and Pain, V.M. (1993). Eur. J. Biochem., 218, 39-48. [3]. Rau, M., Ohlmann, T., Morley, S.J. and Pain, V.M. (1996). J. Biol. Chem. 271, 8983-8990. [4]. Morley, S.J. and McKendrick, L. (1997). J. Biol. Chem. 272, 17887-17893. [5]. Morley, S.J. (1997). FEBS Lett. 418, 327-332. [6]. Fraser, C.S., Pain, V.M., and Morley, S.J. (1999) J. Biol. Chem. 274, 196-204. [7]. Fraser, C.S., Pain, V.M., and Morley, S.J. (1999). Biochem. J. 342, 519-526. [8]. Morley, S.J., and Naegele, S. (2003) Cell. Signalling 15, 741-749. [9]. McKendrick, L., Morley, S.J., Pain, V.M., Jagus, R., and Joshi, B. (2001) Eur. J. Biochem. 268, 5375-85. [10]. Morley, S.J., and Naegele, S. (2002) J. Biol. Chem. 277, 32855-32859. [11]. Naegele, S., and Morley, S.J. (2004) J. Biol. Chem. 279, 46023-46034. [12]. Ling, J., Morley, S.J., Pain, V.M., Marzluff, W.F., and Gallie, D.R. (2002) Mol. Cell. Biol. 22, 7853-7867. [13]. Goggin, M.M., Nelsen, C.J., Kimball, S.R., Jefferson, L.S., Morley, S.J., and Albrecht, J.H. (2004) Hepatol. 40, 537-544. [14]. Cowan, J.L., and Morley, S.J. (2004) Eur. J. Biochem. 271, 3596-3611. [15]. Ling, J., Morley, S.J., Traugh, J.A. (2005) EMBO J. 24, 4094-105. [16]. Coldwell, M.J, and Morley, S.J. (2006). Mol. Cell. Biol. 26, 8448-8460. [17]. Hinton, T.M., Coldwell, M.J., Carpenter, G.A., Morley, S.J., and Pain, V.M. (2007). J. Biol. Chem., 282, 1695-708. [18]. McKendrick, L., Thompson, E., Ferreira, J., Morley, S.J., and Lewis, J.D. (2001) Mol. Cell Biol. 21, 3632-41. [19]. Woods, A.J., Roberts, M.S, Choudhary, J., Barry, S.T., Mazaki, Y., Sabe, H., Morley, S.J., Critchley, D.R. and Norman, J.C. (2002) J. Biol. Chem. 277, 6428-6437. [20]. Coldwell, M.J., Hashemzadeh-Bonehi, L., Hinton, T.M., Morley, S.J., and Pain, V.M. (2004) J. Cell Sci. 117, 2545-2555. [21]. Ohlmann, T., Rau, M., Morley, S.J., and Pain, V.M. (1995). Nucleic Acids Res. 23, 334-340. [22]. Ohlmann, T., Rau, M., Pain, V.M. and Morley, S.J. (1996). EMBO J. 15, 1371-1382. [23]. Clemens, M.J., Bushell, M., and Morley, S.J. (1998). Oncogene 17, 2921-2931. [24]. Bushell, M., Wood, W., Clemens, M.J., and Morley, S.J. (2000). Eur. J. Biochem. 267, 1083-1091. [25]. Bushell, M., McKendrick, L., Janicke, R.U., Clemens, M.J., and Morley, S.J. (1999). FEBS Lett. 451, 332-6. [26]. Bushell, M., Poncet, D., Marissen, W.E., Flotow, H., Lloyd, R.E., Clemens, M.J., and Morley, S.J., (2000). Cell Death Differ. 7, 628-636. [27]. Morley, S.J., McKendrick, L., and Bushell, M. (1998). FEBS Lett. 438, 41-48. [28]. Bushell, M., Wood, W., Carpenter, G., Pain, V.M., Morley, S.J., and Clemens, M.J. (2001) J. Biol. Chem. 276, 23922-23928. [29]. Spriggs, K.A., Cobbold, L.C., Jopling, C.L., Cooper, R.E., Wilson, L.A., Stoneley, M., Coldwell, M.J., Poncet, D., Shen, Y.C., Morley, S.J., Bushell, M., Willis, A.E. (2009) Mol. Cell. Biol. 29,1565-74. [30]. Willett M, Cowan J.L., Vlasak M., Coldwell M.J., and Morley S.J. (2009) Cell Signal. 21,1504-12. [31]. Willett M, Pollard H.J., Vlasak M., Morley S.J. (2010) Biol Cell 102, 265-76
Aims of the current work
The aims of my current work are to investigate the signalling pathways regulating mRNA utilisation in eukaryotic cells during proliferation and differentiation. My previous studies have clearly shown the co-ordinate phosphorylation of components of the translational machinery has a central role in the regulation of protein synthesis, and may mediate the selection of specific mRNA species for translation. One of these components, which is the main focus of my work, is the initiation factor complex, eIF4F. Increased phosphorylation of two components of eIF4F (eIF4E and eIF4G) is an early response to stimulation of translation by a wide variety of agents (serum, hormones, mitogens). We are currently investigating the assembly of this complex during different phases of the cell cycle and the control of complex assembly during myogenic differentiation. Although the regulation of protein synthesis is fundamental to cell growth and/or survival, relatively little is actually known about the role of phosphorylation of translation initiation factors in modulating this process. In particular, there is, as yet no direct evidence that phosphorylation of eIF4E or eIF4G at physiological sites has any effect on translation rates or cell growth in vivo.
In light of my previous findings, we are currently:
(a) investigating the signalling pathways utilised to modulate eIF4F phosphorylation and rates of protein synthesis in vivo, with particular emphasis on the role of the eIF4E kinase, Mnk1;
(b) determining how the phosphorylation status of eIF4E and other RNA binding proteins influences the selection of specific mRNAs for translation;
(c) investigating the role of intracellular localisation of initiation factors in translational control;
(d) investigating the role for initiation factor phosphorylation in controlling cellular differentiation.
(e) initiating new links with the BSMS to pioneer studies into the role for translational control in leukaemic stem cell proliferation (Dr. T. Chevassut), wound healing (Dr. S. Newbury) and loss of growth control in glioma cells (Dr. A Chalmers).
We have been addressing these related questions by parallel studies using human, mouse and Xenopus cells in culture. We believe that these studies will substantially increase our understanding of the significance of the control of protein synthesis in the regulation of cell growth or death.