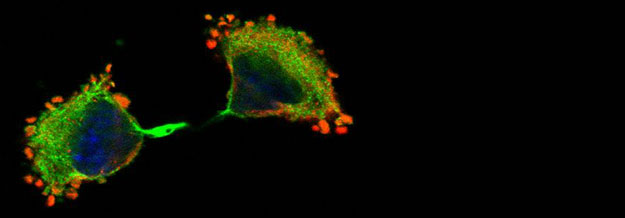
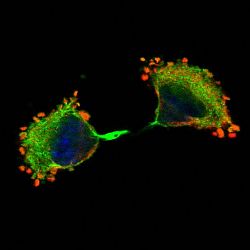
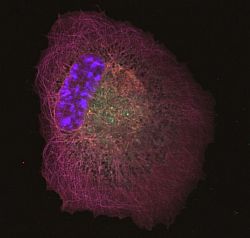
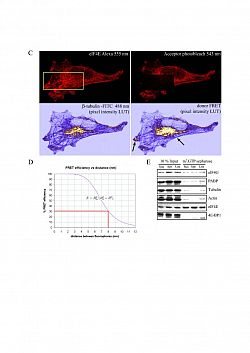
We are investigating the signalling pathways regulating mRNA utilisation in eukaryotic cells during proliferation and differentiation. Our main focus is on the initiation factor complex, eIF4F, and its regulated assembly during different phases of the cell cycle. We are also developing tools to investigate localised protein synthesis in cells maintained in 2D and 3D culture. Although the regulation of protein synthesis is fundamental to cell growth and survival, relatively little is actually known about the role of phosphorylation of translation initiation factors in modulating this process.
Current research interests
Importance
The initiation stage of translation is a major site of regulation of gene expression with strong analogies to the assembly of transcription complexes at promoter sites on DNA. In terms of regulatory significance, the second phase of initiation, the binding of mRNA to the ribosome, has the potential to modulate both the overall rate of protein synthesis and the selective recruitment of specific mRNAs for translation. This step can be modulated by both the phosphorylation of key initiation factors and by changes in the levels of these factors. The eIF4 initiation factors (eIF4E, eIF4A, eIF4B, eIF4G and the eIF4F complex) are responsible for recruiting mRNA to the ribosome and our work is focused on the activity of these proteins.
In summary, eIF4E interacts specifically with the mRNA cap structure and is essential for cap-dependent translation. eIF4A exhibits RNA-dependent ATPase and bidirectional RNA helicase activities,with the latter strongly enhanced by eIF4B. eIF4G, of which there are two forms (eIF4GI, eIF4GII), functions as an adapter protein in the assembly of the initiation complex, eIF4F. It binds, via a defined and conserved sequence, to the convex surface of eIF4E and has a centrally located domain which interacts with eIF3 and eIF4A, with consensus elements characteristic of RNA binding proteins; both mammalian eIF4G homologues possess an N-terminal binding site for poly(A) binding protein (PABP). A number of independent and cross-talking signalling pathways have the potential to regulate the utilisation of localised mRNAs by the translational machinery. eIF4E, the mRNA cap binding protein undergoes regulated phosphorylation on Ser209, mediated via multiple signalling pathways distinct from mammalian target of rapamycin (mTOR). Physiologically regulated protein kinases, Mnks, which interact directly with the scaffold protein eIF4G, act at the convergence point of the ERK1/2 and p38MAP kinases to phosphorylate and regulate the activity of eIF4F. This event is required for tumourigenesis in vivo. The association of 4E-BPs with eIF4E is acutely modulated by multi-site phosphorylation events that are dependent upon mTORC1 signalling. This process effectively integrates signals from mitogens and nutrients with the translational apparatus. In a poorly understood process, these effects can also be localised to specific regions of a cell.