Gray et al. Open Biology 3(7):130019. doi:10/1098/rsob.130019
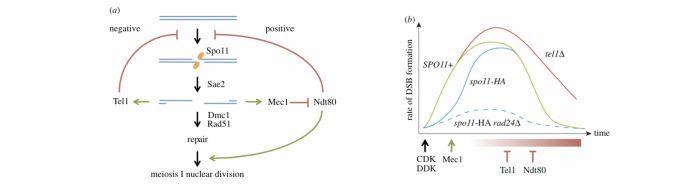
During meiosis, DSB formation and repair must be tightly temporally regulated: DSBs must arise after genomic regions have been replicated, and DSB repair must be completed prior to the onset of chromosome segregation. Once DSBs begin to form, ssDNA present in transient recombination intermediates are coated with replication protein A (RPA), leading to recognition, binding, and signalling by the evolutionarily conserved checkpoint kinase ATR (Mec1 in S. cerevisiae). In S. cerevisiae, the resulting protein phosphorylation cascade leads to transient cell cycle arrest mediated by the kinase Mek1, a paralogue of Rad53/CHK2. Loss of Mec1 signalling generates inviable aneuploid spores, in part due to an increase in inter-sister recombination (DSB repair normally displays a bias toward recombination between homologous chromosomes), and in part due to a failure to complete DSB repair prior to chromosome segregation. As expected for a factor activated by ongoing DSB repair, all preceding work had suggested a role for the Mec1 (and Mek1) kinase downstream of DSB formation. In the work described by Gray et al., we demonstrate for the first time an upstream role for Mec1 activation in promoting formation of meiotic DSBs via its effects on delaying cell cycle progression.
Our work arose from the intriguing observation that the abundance of Spo11-oligo complexes (intermediates in DSB repair) and DSBs themselves (as measured physically by Southern blotting of specific DSB loci and along whole chromosomes) were reduced when Mec1 signalling was perturbed (achieved via mutation of the checkpoint clamp loader, Rad24/RAD17), only when Spo11 was conjugated to an epitope tag known to moderately reduce its catalytic activity (Spo11-HA). These synergistic reductions in DSB formation correlated with synergistic reductions in spore viabilty and more rapid progression through meiotic prophase. Because these effects were dependent on the hypomorphic spo11 allele, we hypothesised that Mec1 activity becomes critical to achieve maximal DSB formation when Spo11-DSB formation itself is less efficient.
To test this idea, we engineered strains that would arrest permanently and/or transiently during meiotic prophase independently of Mec1 activity. Remarkably, permanent prophase arrest led to a restoration of high levels of Spo11-DSB formation in the Spo11-HA rad24∆ strain, indicating that the previous loss of DSBs was due to rapid exit from meiotic prophase. Moreover, even transient prophase arrest in the absence of full Mec1 activity was sufficient to restore high levels of spore viability. Notably, transient arrest of the single rad24∆ mutation also improved spore viability (even in the absence of any secondary defect in Spo11), suggesting that part of the reason for spore lethality in rad24 and mec1 strains is due to insufficient time for DSB formation and/or repair prior to chromosome segregation.
Collectively, our work, along with that of others recently published (Argunhan et al, PLoS One 2013; Thacker et al, Nature 2014; Garcia et al, Nature 2015), have aided to elucidate that the regulation of meiotic DSB formation involves a complex set of overlapping feedback pathways, where even factors classically thought to function downstream can have upstream effects (in trans) at other sites within the nucleus due to the asynchronous and variable distribution of meiotic recombination initiation that arises across the genome (reviewed by our team in Cooper et al, Experimental Cell Research 2014).
References: