Background
In addition to the conventional cytochrome c oxidase, plant mitochondria contain a non-protonmotive alternative oxidase (AOX) that couples the oxidation of ubiquinol directly to the reduction of molecular oxygen. In thermogenic plants, AOX is responsible for heat generation, whilst in non-thermogenic species, the oxidase is thought to play a more fundamental role in the regulation of energy metabolism. AOX may be involved in facilitating TCA cycle turnover, protection against oxidative stress, and preservation of plant growth homeostasis (see [1, 2] for review). AOX proteins are not restricted to plants, however, but also occur in many fungi [3] as well as several pathogenic organisms including Trypanosoma brucei (which causes African Sleeping Sickness) [4], intestinal parasites such as Cryptosporidium parvum and Blastocystis hominis [5, 6] and opportunistic human pathogens such as Candida albicans [7]. Because of their absence in the mammalian host, AOX proteins are potential therapeutic targets in these systems.It should be noted that immunocompromised individuals are particularly susceptible to these opportunistic human diseases and new drugs that are well tolerated and have clearly defined biochemical targets are therefore urgently required [8, 9].
The current structural model predicts that AOX is an integral (~32 kDa) interfacial membrane protein that interacts with a single leaflet of the lipid bilayer, and contains a non-haem diiron carboxylate active site [10-12]. This model is supported by extensive site-directed mutagenesis studies [13, 14]. Moreover, EPR and FTIR spectroscopic experiments have confirmed the presence of a binuclear iron centre [15-17].
The research being undertaken in my laboratory aims to investigate one such target, namely the alternative oxidase, with the research outcomes not only providing new insights into the mechanism and inhibition of the alternative oxidases but ultimately the research may translate into practical applications since such information will assist in the suitable rational design of phytopathogenic fungicides and anti-parasitic drugs that are specifically targeted to the alternative oxidase since the protein is not found in humans.
Research Challenges
A key challenge in understanding how to control the alternative oxidases (AOX) in plants, fungi and parasites rests upon the identification of the substrate-binding site in this protein. A detailed knowledge of the nature of this binding site is important since it will reveal whether or not there is a common architecture that can be applied to quinol-binding sites in general, will provide an insight into the mechanism of binding and importantly will result in rational drug design.
Research Interests and Strategy
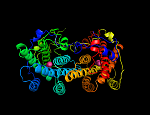
In our recently completed BBSRC-funded programme of work, we were able to identify the quinol-binding site of the plant AOX [18, 19], and solve the first crystal structure, in collaboration with out Japanese colleagues (Prof. Kiyosih Kita, University of Tokyo), of an AOX to greater than 2.9Å resolution [20-23].
A knowledge of the structure of AOX now allows us to probe the active-site, and the substrate and inhibitor binding site(s) of the plant and trypansomal alternative oxidases in more detail through the structural and molecular characterisation of recombinant and mutant forms of AOX in the following manner:
(i) site-directed mutagenesis to generate a number of site-specific mutant AOXs possessing mutations in the active-site and the substrate and inhibitor binding domain;
(ii) kinetic characterisation of specific inhibitors using polarographic and voltametric techniques on both wt and mutant forms of AOX;
(iii) the design and synthesis of new AOX inhibitors with Prof. Simon Ward (University of Sussex);
(iv) spectroscopic characterisation of the substrate/inhibitor binding sites using FTIR, EPR and ENDOR and ESEEM techniques with Professors Peter Rich (University College London) and Peter Heathcote (Queen Mary College, University of London)
(v) structural characterisation (crystallography) of wt and mutant rAOX in the presence of substrate and inhibitors with Professor So Iwata (Imperial College, London) and Professor Kiyoshi Kita (University of Tokyo, Japan).
(vi) molecular and developmental characterisation of Arum maculatum alternative oxidases during thermogenesis with Prof. Ito (University of Iwate, Japan)
Recent Results
1.The first FTIR study of the redox centres of a highly purified and stable preparation of recombinant AOX (rTAO) from Trypanosoma brucei which confirmed the presence of a diiron centre. This study revealed clear spectroscopic signatures of two distinct redox processes associated with the full reduction of oxygen through to water. Spectroscopic signatures contained features that could be assigned to the protonation of at least one carboxylate group and further perturbations of carboxylic and histidine residues. Furthermore these spectra open the way for a detailed characterisation of the redox reactions, thermodynamics, inhibition by ascofuranone and other inhibitors and the catalytic cycle of oxygen reduction by AOXs. Publication no. 17
2.The discovery that Q242, S256, H260 and R261, residues we had previously proposed to be implicated in substrate/inhibitor binding, are absolutely critical for Sauromatum guttatum AOX (SgAOX) activity. Of particular importance was the finding that within the quinol-binding pocket N247 played a critical role in inhibitor sensitivity. Publication no. 18
3.Generating a T179A mutant which has an exclusive and significant affect upon the Km for oxygen of the alternative oxidase (from 18 to 5 µM). EPR spectroscopy has revealed that the T179A mutant traps a stable ubisemiquinone in the ubiquinol-binding site. ENDOR spectroscopy is currently providing significant insights into the ubiquinol-binding site. Publication no.19
4.The development of techniques for the production of electrophoretically pure AOX enzyme with high specific activity and excellent yield which has been used to generate the first ever trypanosomal AOX crystals which diffract to greater than 2.8 Å resolution in the presence and absence of inhibitors. Publication nos. 20, 21, 22
Documentation: [1] Affourtit et al. (2002) FEBS Lett. 510, 121. [2] Moore et al. (2002) Trends in Plant Science 7, 478. [3] McDonald &, Vanlerberghe (2006) Comp Biochem Physiol D 1, 357. [4] Chaudhuri et al. (2006) TiParasitol 22, 484.[5] Roberts et al. (2004) Int J Parasitol 34, 297. [6] Suzuki et al. (2004) Biochem. Biophys. Res. Commun. 313, 1044.[7] Yan et al. (2009) J. Antimicrob. Chemo. 64, 764. [8] Cross (2010) Nature 464, 689 [9] Frearson et al (2010) Nature 464, 728. [10] Moore &, Albury (2008) Biochem Soc Trans 36, 1022. [11] Andersson &, Nordlund (1999) FEBS Lett 449, 17. [12] Berthold et al. (2000) Biochim. Biophys. Acta 1460, 241. [13] Albury et al. (2002) J. Biol. Chem. 277, 1190. [14] Albury et al. (1998) J. Biol. Chem. 273, 30301. [15] Berthold et al. (2002) J Biol Chem 277, 43608. [16] Moore et al. (2008) Biochim. Biophys. Acta 1777, 327 [17] Maréchal et al. (2009) J. Biol. Chem. 284, 31827 [18] Albury et al. (2010) Biochim. Biophys. Acta 1797, 1933 [19] Crichton et al. (2010) Biochim. Biophys. Acta 1797, 732. [20] Kido et al. (2010) Biochim. Biophys. Acta 1797, 443. [21] Kido et al. (2010) Acta. Cryst. F66, 275. [22] Shiba et al. (2011) In preparation