Website Research Statement
Rationale.
Change is one of Nature’s constants. Physical change, such as wind, rain, snow, and chemical change,– rock weathering, rusting of iron, etc. all result from molecular collisions. Can we understand these events at the molecular level? Can we predict them? Can we control them? In the McCaffery lab, collisions have been studied in exquisite experimental detail over a number of years, so that now, understanding collisions is routine and prediction straightforward in many instances. However, to get to this level, and eventually proceed beyond, it was necessary to develop a new theoretical approach to collision-induced change. At molecular level, change involves transitions between quantum states in the collision partners and this forms an essential element in the new theory.

A new theory.
Our experiments, together with complementary work by other groups, indicated strongly that momentum change provides the motive force for physical and chemical change. This differs from conventional collision theory in which energy change provides the driving force. In the new approach, we calculate the probability of converting linear momentum of collision into angular momentum (rotational and recoil orbital) of the products. Energy conservation is enforced requiring each colliding molecule to undergo a transition between quantum states, i.e. there is state-to-state and overall energy conservation. The method, called the angular momentum (AM) theory, is simple and transparent. It is readily computerised and now calculations are fast and accurate, requiring only readily available data such as bond lengths, atomic mass and spectroscopic constants.

Research Projects.
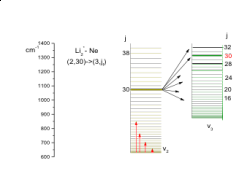
The AM theory is used to interpret data from numerous experiments, giving new insight into the underlying mechanisms. Collaboration with the research groups of Prof. Warren Lawrence (Flinders University, Adelaide) and Prof. Hanna Reisler (University of Southern California) has led to understanding of the factors that control vibrational energy transfer in polyatomic molecules and the dissociation of weakly-bonded acetylene – HX complexes. In both instances, the angular momentum ‘gap’ is a key controlling factor as the probability of generating AM falls exponentially with AM magnitude. This AM gap problem can be overcome via a suitable energy ‘sink’ such as an accessible vibrational state that reduces the angular momentum load and accelerates energy transfer or dissociation.
Basic equations: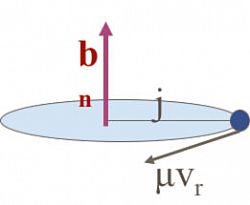
1. Δj = μ vr bn (LM à AM)
2. ½μ vr2 = ΔE =|Ef –Ei|
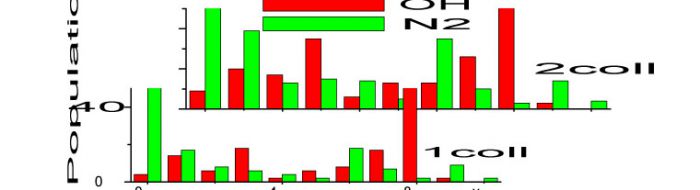
Most recently the speed and efficiency of the AM method has been used to construct a computer-model of the flow of energy in gas ensembles containing up to 10,000 molecules. This first-generation model of this revolutionary approach to treating real-life gas phase systems is currently limited to atoms and diatomic molecules, though can deal with mixtures containing up to three different diatomics. The next version, now being developed, will include new features including extension to include polyatomic molecules. The program computes state-to-state collision-induced energy transfer among the 10000 molecules as they undergo many thousands of collision cycles, each cycle comprising 10000 randomly selected pairs of molecules sequentially to undergo binary collisions.
The primary data from the program are the quantum state populations, which are available after each collision cycle. In addition, results are presented as the modal temperatures of vibration, rotation and translation for each of the species present. Examples of these two outputs are shown below for 14N2 in rovibrational state v8; j10 and present as the minor component in a bath of 15N2 in v0; j10. The figures show both the evolution of modal temperatures as a function of number of collision cycles and the change in vibrational state populations after 1, 5 and 10 collision cycles.
Current research centres on the role of collisional energy transfer in Earth’s atmosphere and impact on global warming. The major constituents of the atmosphere, N2 and O2 are unable to absorb or radiate electric dipole radiation in the infra-red or microwave regions and much of the debate centres on the radiation balance involving minor components such as CO2, H2O etc. Little is known of the extent to which collisions influence this process to populate or depopulate the molecular radiators. It is likely that collisional transfer rates for the minor species greatly exceed those resulting from radiative population and loss of terrestrial infra-red. A lengthy study of energy flow between the major and minor components of Earth’s atmosphere has begun with the aim of quantifying the role of collisions in global warming.
Other, longer term, research projects involve use of the multi-collision model in a range of applications including a number of astrophysical contexts, in modelling gas lasers with the aim of predicting new potential gain media and developing criteria for optimising gas laser efficiency and in developing models of e.g. combustion and industrial plasmas more widely.