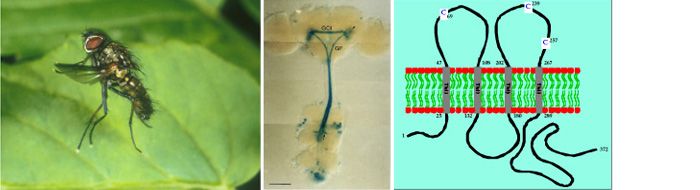
The large descending Giant Fibre (GF) integrates visual and antennal input in the brain and rapidly conveys action potentials to the thoracic ganglion, where the GF forms an electrical synapse with the Tergotrochanteral Motoneuron (TTMn) which innervates the ‘jump muscle’ (TTM) of the middle leg.
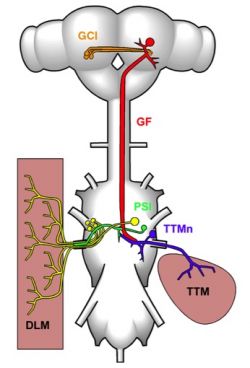 Drosophila Giant Fibre System. A schematic drawing showing the central nervous of the fly, and the neurons and muscles of the GFS. In the brain, the Giant Fibre (GFs; red) forms synaptic connections with the Giant Commissural Interneurons (GCIs; orange). The GFs descend to the mesothoracic neuromere of the thoracic ganglion where they synapse directly with the motorneurons (TTMn; blue) of the tergotrochanteral muscle (TTM; pink, right) and indirectly, via the peripherally synapsing interneuron (PSI; green), with the motorneurons (DLMns; yellow) of the dorsal longitudinal muscles (DLM; pink, left). Note that, for clarity, only one of the TTMns and one set of DLMns, and the corresponding neuromuscular junctions, are shown.
Drosophila Giant Fibre System. A schematic drawing showing the central nervous of the fly, and the neurons and muscles of the GFS. In the brain, the Giant Fibre (GFs; red) forms synaptic connections with the Giant Commissural Interneurons (GCIs; orange). The GFs descend to the mesothoracic neuromere of the thoracic ganglion where they synapse directly with the motorneurons (TTMn; blue) of the tergotrochanteral muscle (TTM; pink, right) and indirectly, via the peripherally synapsing interneuron (PSI; green), with the motorneurons (DLMns; yellow) of the dorsal longitudinal muscles (DLM; pink, left). Note that, for clarity, only one of the TTMns and one set of DLMns, and the corresponding neuromuscular junctions, are shown.
In a major collaboration with Jane Davies (Sussex) and Pauline Phelan (Kent) https://www.kent.ac.uk/bio/profiles/staff/phelan.html, we have examined behavioural mutants in Drosophila, isolated because of their inability to escape in response to a visual stimulus. In flies carrying the shaking B2 mutation (previously known as Passover), the electrical synapse between GF and TTMn fails to develop normally, while leaving the propagation of action potentials unaffected. Anatomical studies show that the gross morphology of the GF and TTMn in the mutant is normal and the two neurons still come into cell-cell contact, but dye coupling is disrupted between these neurons. This indicates that the shaking B gene is required for electrical synapse formation in this system. Expression of Shaking B in Xenopus oocyte pairs shows that Shaking B proteins are functional components of gap junctions. We have called this family of gap-junction proteins the Innexins. Drosophila has 8 innexin genes coding for at least 10 different proteins.
But which Innexin proteins form electrical synapses in the Giant-Fibre System? The shaking B locus encodes three members of the innexin family: Shaking B(neural), Shaking B(n+16) and Shaking B(lethal). It seems that electrical synapse formation between the GF and the TTMn probably requires the presence of different Innexins on the two sides of the synapse; in situ hybridization reveals that shaking B(n+16) is the only shaking B transcript expressed in the presynaptic GF (Zhang et al 1999, J Neurobiol 40:288-301), but is not detectable postsynaptically in the TTMn. This motoneuron, however, almost certainly expresses shaking B(lethal).
It has been shown previously that the mutation shaking B2, which removes both Shaking B(neural) and Shaking B(n+16) function, disrupts dye coupling between the GF and TTMn. Targeted expression of Shaking B(n+16) to the GF and TTMn is sufficient to restore dye coupling in these mutants. However expression of Shaking B(neural), identical to Shaking B(n+16) but for the absence of the first 16 amino acids, fails to afford rescue. The most parsimonious explanation for these data is that there is a requirement for different Innexins on the two sides of the synapse - Shaking B(n+16) presynaptically in the GF and Shaking B(lethal) postsynaptically in the TTMn - for electrical synapse formation between these neurons. Supporting this proposal, the in vitro expression of ShakB(n+16) and ShakB(lethal) in neighbouring Xenopus oocytes results in the formation of heterotypic intercellular channels which are asymmetrically gated by transjunctional voltage and exhibit classical rectification. These data provide the most definitive evidence, to date, that rectification is achieved by differential regulation of the pre- and post-synaptic elements of structurally asymmetric junctions.
In vertebrates, gap-junction channels were thought to be entirely composed of a different protein family, called connexins, but work (not at Sussex) has shown that functional innexin-like proteins, termed pannexins, are also expressed in vertebrates. These findings have led us to seek, and determine, the evolutionary provenance of gap-junction proteins; the simple diploblastic organism Hydra appears to possess only innexins. We conclude that innexins are the primordial gap-junction molecules, while connexins evolved more recently in the deuterostomes.