Professor Geoff Cloke FRS
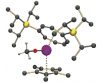 Uranium methoxide complex forms from CO and H2
Uranium methoxide complex forms from CO and H2
Our major research interest in the energy area is on the development of new approaches to the activation and functionalisation of carbon monoxide and carbon dioxide. In particular we are looking at the reductive assembly of carbon monoxide or carbon dioxide on low oxidation state metal centres (e.g. U(III) and Zr(III)) to build simple organic molecules and rings stoichiometrically, and ultimately catalytically. The hydrogenation of CO2 or CO to methanol (thus effectively closing the carbon cycle) under mild conditions is also a major goal, which we have achieved in part as shown on the right.
For more information visit the Cloke Lab website
Dr John Turner
For more information visit the Turner Lab website
Dr Hazel Cox
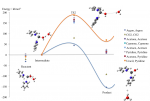 Moderating the acidity of Pb(II)-Water complexes through the coordination of non-aqueous ligands
Moderating the acidity of Pb(II)-Water complexes through the coordination of non-aqueous ligands
In our research we use quantum chemistry to understand fundamental aspects of the behaviour of metal ions in solution by determining the underlying chemical and physical reasons for the stability, reactivity and spectroscopy of multiply charged metal-ligand complexes in the gas phase. By mimicking the immediate environment of the metal ion with a limited number of ligands we can use it to explain other patterns of behaviour, for example, the inherent (Lewis) acidity of metal ions. Theory contributes significantly both to the development and interpretation of new gas phase experiments and to testing new and standard quantum techniques on solvated metal ions. A further objective is to identify areas where developments in the methodology are required.
For more information visit the Cox Lab website
Dr QIao Chen
 Zinc oxide nano rods for water splitting
Zinc oxide nano rods for water splitting
Our long term research interests are on the nanochemistry and nanotechnology. The application of nanotechnology to biosensor design and fabrication promises to revolutionize diagnostics and therapy at the molecular and cellular level. Our research aims to develop medical, biological and chemical sensors with optoelectronic devices on nanometer length scales. Projects include the synthesis and chemical modification of quantum dots, nano wires and nano tubes and utilizes state of the art nanoscience facilities including scanning electron microscopy, atomic force microscopy and scanning tunneling microscopy.
For more information visit the Chen Lab website
Dr Ian Crossley
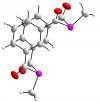 The first diphospha-metacyclophane
The first diphospha-metacyclophane
A major focus of our work is the development of synthetic routes to polyconjugated organometallics bearing low-coordinate phosphorus centres. Conjugated organometallics are widely utilised in efforts to develop molecular electronic and opto-electronic materials; we are seeking to use low-coordinate phosphorus as a means of tuning the electronic response by modifying frontier orbital energies. In other projects we are involved the synthesis and study of novel ligands (right) and molecular scaffolds for the activation of small molecules.
For more information visit the Crossley Lab website
Dr Mark Osborne
 CdSe Quantum Dots from the Osborne Lab
CdSe Quantum Dots from the Osborne Lab
Photo-active nanoparticles, in particular Quantum Dots, are now being used in devices and materials, from photovoltaic cells and low-energy LED lighting and displays, to self-cleaning glass. While quantum confinement effects in these zero dimensional materials have many advantages over conventional photoactive materials, namely the size tunablility of band-edge absorption and emission, surface to volume ratios at the nanoscale mean interfacial defects can have significant and detrimental effects on quantum yields and photostabilities of these particles.
Research in the Osborne Lab aims to understand the photophysics of semiconductor nanocrystals including fluorescence intermittency, photobrightening and bluing with a view to engineering properties through rational synthesis.
For more information visit the Osborne Lab website


