Serpell group focusses on the structure and function of amyloidogenic peptides and proteins
This specific structural conformation is associated with fibrillar deposits in a number of neurodegenerative diseases such as Alzheimers and Parkinsons Disease as well as CJD. Curiously each disease is caused by a different protein and yet each is caused by the folding of the respective protein into amyloid.

As well as these proteins being disease related, nature has found ways to use amyloid to carry out specific roles. These functional amyloids (including yeast prions) have evolved to be tightly controlled and have become essential for epigenetic inheritance, scaffolding for cell matrices and even protecting developing cells. We hope to understand how amyloid could be controlled and used to such an extent that new nano-technologies could stem from these useful fibrous materials.
As a group we work in particular on Amyloid Beta protein and a number of amyloid-like model systems. We use techniques to biophysically and structurally characterise proteins including circular dichroism, intrinsic fluorescence, transmission electron microscopy and experimental and simulated x-ray fiber diffraction.

To bridge the gap between structural characterisation and potential pathological effects, we are exploring the effect of amyloid oligomers on synthetic membranes in leakage assays and on neuronal cell lines.

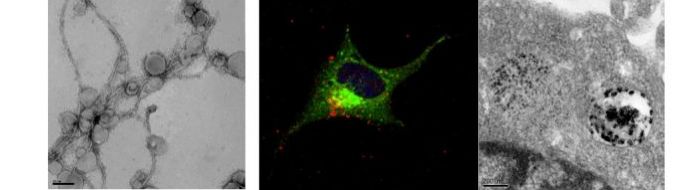
Alzheimer's disease is associated with the accumulation of amyloid fibrils formed by Abeta peptide and by intracellular accumulation of tau in neurofibrillary tangles. Our recent work has concentrated on understand how small assemblies of Abeta known as oligomers, are able to affect neuronal cell survival and their function.
In recent work, we showed that Abeta oligomers cause permeation of lipid membranes, and this may be a mechanism by which Abeta can exert its toxic effect (Williams et al., 2010 and 2012).
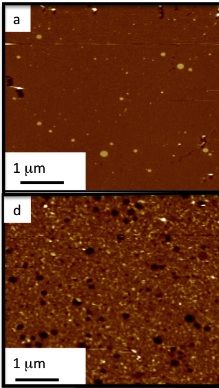 Lipid bilayer under Atomic force microscope (a). F) shows the bilayer treated with Abeta (1-42) oligomers revealing permeation of the membrane
Lipid bilayer under Atomic force microscope (a). F) shows the bilayer treated with Abeta (1-42) oligomers revealing permeation of the membrane
Treatment of neuroblastoma cells and primary neurons with Abeta oligomers results in deterioation of the cells and eventually cell death. Using confocal and electron microscopy, we were able to show that Abeta oligomers enter cells via an endocytotic mechanism and to accumulate in lysosomes and autophagosomes.
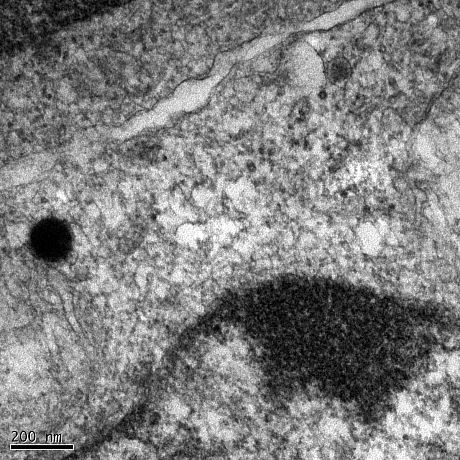
Neuroblastoma cell treated with Abeta(1-42) oligomers for 24hours