Research sheds new light onto quantum dots and their applications in nanotechnology
By: Jessica Gowers
Last updated: Friday, 14 June 2019
Graphic representation of the exciton electron and hole wavefunctions in a quantum dot
Research by chemists at the University of Sussex has revealed new factors that influence blinking in Quantum Dots (QDs).
It is expected the research will help towards enhancing QD performance in display technology, such as televisions, solar cells and more.
QDs are tiny semiconductor particles, only a few nanometers in size and can be made up of a variety of elements. Their size and energy level can be precisely controlled, which makes them useful for a wide range of applications.
Their luminescent properties also make them desirable in a range of disciplines; when irradiated with a UV light, different sized QDs emit different colour lights.
The paper, published in Nature Communications Chemistry, analyses the effects of ligands (ions/molecules that bind to a central metal atom) on this QD photoluminescence.
Dr Mark Osborne looks behind the research:
“To illuminate a little on the title. The work isn’t going to change the world, but in recognition of the ‘The International Year of the Periodic Table’ the nanomaterials we study in the paper contain cadmium and selenium, elements found in Groups 12 and 16 of Dimitri Mendeleev’s masterpiece. Perhaps more importantly is that when we bring the metal and non-metal together in small amounts we form quantum dots (QDs), the bit of tech that now gives QLED TVs near-perfect colour reproduction (I know, with skin in the game, I couldn’t not get one!). They also give solar cells a bit more oomph! By small, we mean these CdSe nanocrystals are synthesised only a few 0.000000001 metres in diameter. To give a sense of just how small, the QD is to a tennis ball, what the tennis ball is to the Earth, but they pack a punch when it comes to emitting light and can be tuned to fluoresce brightly at chosen colour in the rainbow, simply by changing the size of the “quantum box”, viz. the nanocrystal.
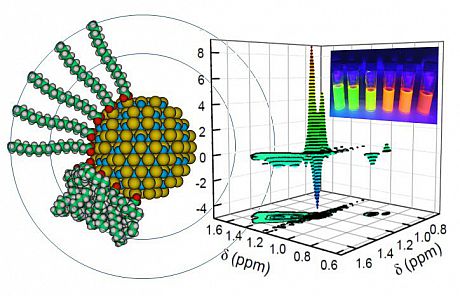
“Model of CdSe quantum dot with organic ligand in extended and collapsed conformations on the QD surface (Cd = cyan, Se = yellow, C = green, O = red, H = white). NOE spectrum (the Nuclear Overhauser Effect) resolves free ligand (positive signal) from surface bound ligand (negative signal). Inset size tuned QD fluorescence.
“However, these little fellows can suffer from blinking in the fluorescence they emit, switching on and off like a flashlight and this reduces the overall efficiency of emission for an ensemble of QDs, as used in a QLED pixel for example. This is just the kind of “blinking” problem our lab likes to understand, so we looked at the nature of the fluorescence intermittency in single QDs and the factors that control the light-switch. The QD is not isolated but wrapped in a shell of organic ligand molecules that stabilise the CdSe nanocrystal; a sphere of influence that the current paper explores. We used Nuclear Magnetic Resonance (like MRI) spectroscopy to determine the coverage of the ligand on the QD surface and showed this to be consistent with our QD-charging models of fluorescence blinking that support a dominant role of the ligand-shell in governing the duration of bright, emitting periods of the QD. Of course, more QDs switched-on for longer make for more efficient displays. We hope that shining a little light on the factors that influence blinking in QDs will help towards enhancing their performance in display tech, solar cells and other QD applications.”
Measurement of ligand coverage on cadmium selenide nanocrystals and its influence on dielectric dependent photoluminescence intermittency is by Aidan A. E. Fisher, Mark A. Osborne, Iain J. Day and Guillermo Lucena Alcalde.
For more information, visit the Osborne Lab website.