Sponsorship Sub-Committee
The University's Sub-Committee has formal responsibility for oversight of all University of Sussex sponsored research.
SSC Committee dates | Covid-19 requirements
Interim Sponsorship Sub-Committee Chair: Dr Ruth Stirton
Please note that the University reserves the right not to sponsor studies when it believes the proposed project constitutes a risk to the organisation on patient safety, financial or legal grounds.
The Sponsorship Sub-Committee (SSC) has responsibility for formal oversight to all forms of sponsored research
- acceptance of Sponsorship
- amendment requests
- protocol deviations/ breaches of Good Clinical Practice (GCP)
- Serious Adverse Event (SAE) notifications
- reports to NHS Research Ethics Committees (RECs)
Researchers wishing to submit to the Sponsorship Sub-Committee should ensure they check the meeting and submission dates in good time to plan their research activity. Please contact researchsponsorship@sussex.ac.uk
* Should you wish to receive sponsorship for a study, please ensure that you understand how both the PSRP and the Sponsorship Sub-Committee Operate *
When is it appropriate for the University to Sponsor?
For all research projects undertaken by a member of University of Sussex staff or student registered at the University of Sussex (including BSMS) for which no prior agreements have been established that a NHS Trust is more appropriate to sponsor.
- For staff, this will be dictated by the substantive employer.
- For students, it should be the organisation responsible for the qualification being studied.
The University is not usually able to give Sponsorship approval to Clinical Trials of Investigational Medicinal Products (CTIMPs) unless managed by a Clinical Trials Unit (CTU).
All researchers who have been granted University Sponsorship must follow the University's Conditions of Sponsorship Agreement.
The documents indicated for PSRP review are the minimum required to ensure sponsorship review. The University of Sussex SSC also requires in addition:
- After PSRP recommendation: A letter to the Chair of the Sponsorship Sub-Committee with an overview of the study indicating in a numbered list how each of the points raised by the PSRP has been addressed. Documents that have been amended in response to review should feature tracked changes and updated version control.
To avoid delays in receiving University sponsorship, please submit to researchsponsorship@sussex.ac.uk at the same time as sending to psrp@sussex.ac.uk
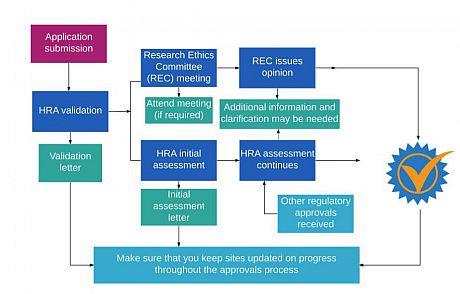
Applicants are strongly advised to read carefully the HRA applicant guidance to understand the subsequent processes to be followed and the documents needed to support an application.
PSRP and formal acceptance of Sponsorship
Once the PSRP has recommended your application to the Sponsorship Sub-Committee for formal University of Sussex sponsorship to be granted (and a letter has been issued to confirm this), you will be able to proceed with gaining HRA and NHS Research Ethics Committee approval.
The majority of studies that are reviewed by the PSRP will be accepted for Sponsorship (after receiving recommendation from the Panel) by Chair's Action between SSC meetings. Exceptions to this are:
- Applications for clinical trials of investigational or medicinal products (CTIMPs)
- Applications for research tissue banks (RTBs)
- Basic science studies (or similar) requiring specific medical supervision and oversight and/or those specifically requiring the declared University’s Clinical Trials insurance extension in addition to standard insurance
- Any other studies identified as leading to non-standard risks or additional liabilities to the Sponsor (typically invoking additional insurance or specific risk mitigation measures)
All researchers who have been granted University Sponsorship are required to agree to follow the University's Conditions of Sponsorship Agreement.
Once Sponsorship has been granted
The Chief Investigator will follow HRA processes until formal notification of approval.
The Chief Investigator has the responsibility to ensure that all amendments, Serious Adverse Events, Annual Progress Reports, protocol or GCP breaches and end of study reports are submitted to the Sponsorship Sub-Committee (in addition to requirements stipulated by the HRA or MHRA (CTIMPs). Please see http://www.sussex.ac.uk/staff/research/governance/sponsorship/sponsorshipduties for further details.
All queries should be submitted to researchsponsorship@sussex.ac.uk